NEB-XIIModel QuestionChemistry 2079/2023 SCROLL DOWN FOR SOLUTION
Rewrite the correct options of each questions in your answer sheet.
GROUP - "A "(11x1= 11)
1. Identify the equivalent weight of K2Cr2O7, in the following reaction?
(Cr =52, K=39)
K2Cr207 + 3H2SO4 + 5(COOH) => K2SO4 + Cr2(SO4)3 + 10C02 + 8H20
A) 49
B) 294
C) 98
D) 108
2.In a solution that is at equil librium. what happens to the concentration of H+ ions if the concentration of
OH- ions is increased?
A) The concentration of H+ ions increases
B) The concentration of H+ ions decreases
C) The concentration of H+ ions stay the same
D) It depends on the initial concentration of H+ ions
3. Assuming the rate of a reaction is doubled for every 10°C rise in temperature how many times increases the rate of temperature rises from 10°C to 100°C?
A) 112 times
B) 400 times
C) 512 times
D) 614 times
4. forthe given reactions
i. C + 02- CO2, H= - × KJmol
ii. 2C0 + 02- 2C02, H = - y KJmol
'The enthalpy of formation of CO becomes
A) 2y-x
B) 2x-y
C)(y-2x)/z
D) (x-2y)/z
5. What product would be obtained if red hot copper wire reacts with steam?
A)CuO
B) Cu2O
C) Cu202.
D) Cuo2?
6. For which manufacturing process, Bessemer converter is used?
A) Pig iron
B) Steel
C) Wrought iron
D) Cast iron
7. When Sodium phenoxide reacts with methyl bromide it gives
A) Cresol
B) Toluene
C) benzene
D) Anisole
8. Identify the X in the following reaction:
A) Na›S
B) Sn/HCI
C) LiAIH.
D) Na/Ha
9. Which of the following reagents can be used to distinguish between a phenol and a carboxylic acid?
A) КОН
B) Na
C) NaOH
D) NaHCO3
10. The colorless sweet smelling liquid compound A which exposed in air forms poisonous phosgene and also react with acetone gives sleep-inducing drug. Predict the product when the compound A reduced in a neutral medium?
A) Methylene chloride
B) Methane
C) Ethyne
D) Ethane
11. Oxygen containing organic compounds upon oxidation forms a carboxylic acid as the major organic product with its molecular mass higher by 14 units. Identify the organic compound.
A) A primary alcohol
B) An aldehyde
C) A ketone.
D) A secondary alcohol
Group B
Short answer questions
(8x5= 40)
12. The addition of solution of required concentration in a reaction mixture yields profitable products and saves reactants.
a) A solution of HCI is labelled 2M. Clarify its meaning?
b) In which aspects molarity is different frommolality?
c) List the significance of normality factor in preparation of standard solution?
d) Liquor ammonia kept at a corner of your chemistry lab is 25% (w/w) NHs and its specific gravity is 0.91. Find the molarity of liquor ammonia.
13. a) Write down the differences between rate of reaction and rate constant. (2)
b) For the reaction 2A+B-product, following data were obtained
Find,
i) order with respect to A and B. (1)
ii) the value of the rate constant of the reaction (1)
iii) the rate of reaction when the concentration of A and B is 0.5M and
0.4 M. respectively. (1)
Or
Four metals A, B, C and D react in the following way:
B displaces only A from solution. Only D and displaces hydrogen from IM HI solution. None of the metals will displace C from solution. Answer the followings:
i) Make the activity series of four metals with hydrogen
il) The standard potential for the following electrodes are:
C++ + 2e----> C, E°->0.76V
D+++ +e- ---> D, E°= +0.77V
a)Construct the galvanic cell by pointing out cathode and anode.
b) With IM solution of the ions, what will be EMF of cell?
c) Will the reaction occur: C++ + 2D++-->- C + D+++ Occur? Give reasons.
14. An ammonia solution is added to the sulphate of coinage metal A,the blue Precipitates (B) which appears dissolves in excess of reagent to form deep blue Solution(C). Answer the followings:
a) Identify A, B and C with sequence of chemical reaction.
b) Predict the electronic configuration of this metal A.
c) Select the suitable methods for the purification of metal A?
15. What is meant by d-d electron transitions? List the characteristics of transition metals.
16. Write down the structural formula and IUPAC name of tertiary alcohol with formula CH1o0. How would you apply Victor Meyer's method for the distinction of 1- propanol and 2- propanol ? Explain.
17. An organic compound X reacts with methyl magnesium bromide followed by acidic hydrolysis yields the compound Y. The compound Y On oxidation with acidified KMnO yields Z. All three gives positive iodoform test. Answer the followings:
a) Predict the compounds X, Y and Z with sequence of reaction and give their IUPAC names. (3)
b) Make the product by reacting Z with dilute NaOH?
OR
An organic compound(X) when heated with acetone gives hypnotic and nervous sedatives drugs and form carbonyl chloride when it exposes to air
a) Predict the organic compound (X)
b) Write the reactions for the formation of (X) from ethanol. A
c) Predict compound the new compound by treating (X) withconcnitric acid?
d) Convert (X) into acetylene.
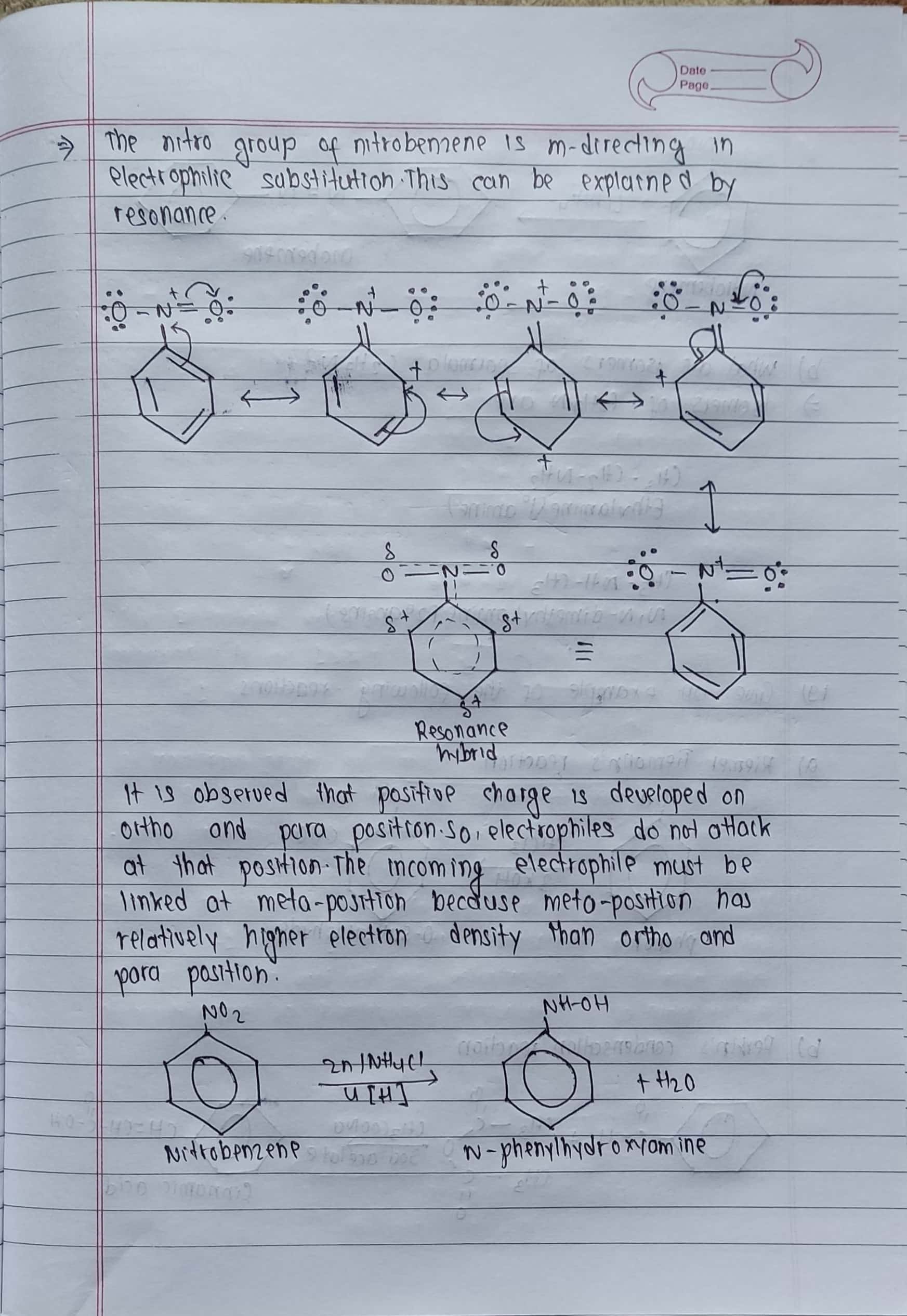
18. a) Nitro group in nitrobenzene is meta-directing group towards electrophilic substitution reaction, why? How does nitrobenzene react with?
i) Zn/ NHACI ii) LiAI4,
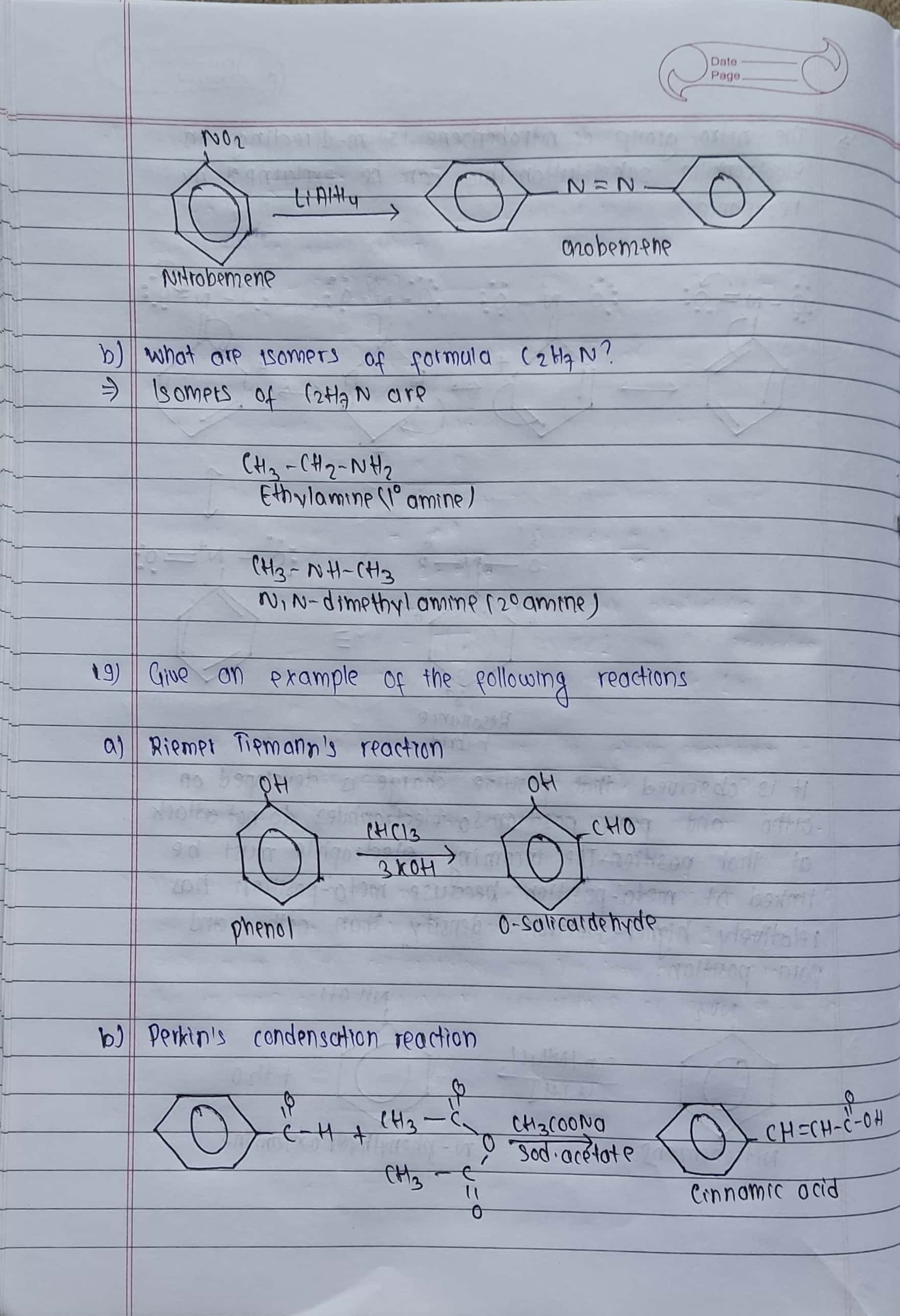
b) What are the isomers of formula C2H7N?
19. Give an example of the following reactions
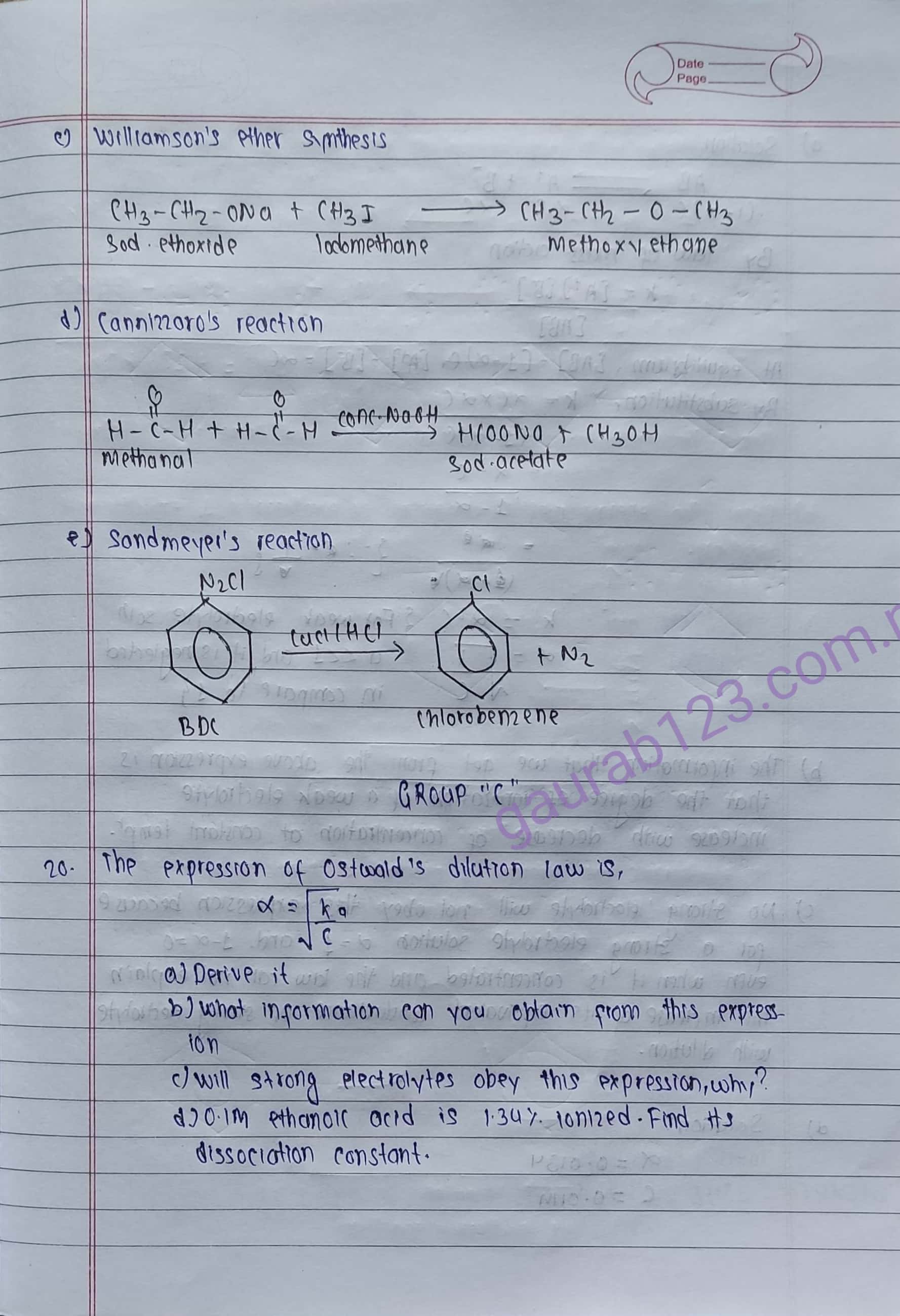
(a) RiemerTiemann's reaction (b) Perkin's condensation reaction
(c) Williamson's ether synthesis. (d) Cannizzaro's reaction
(e) Sandmever's reaction
Group C
Long answer questions
(3 x 8 =24)
20. The expressions of Ostwald's dilution law is, alpha= √k/c
a) Derive it.
b) What information can you obtain from this expression?
c) Will strong electrolytes obey this expression, why?
d) 0.IM ethanoic acid is 1.34% ionized. Find its dissociation constant.
Or
a) Hess' law is applied to calculate different types of enthalpy of reaction.
i) Illustrate the Hass law of constant heat summation.
il) Standard enthalpy of combustion of c (g); He (g) and CH, (g) are -394 KJ mol', -286 KJ mol' and -1300 KJ mol' respectively. Calculate enthalpy of formation of acetylene.
b) Draw the energy profile diagram of exothermic and endothermic reaction.
21. An organic compound C2H4O2 has two functional isomers A and B. Isomer A changes blue litmus into red and B has fruity smell.
a)Give the reaction for the formation of A by using Grignard's reagent.
b) Convert the isomer A into isomerB?
c) Write a suitable test reaction to distinguish A frommethanoic acid.
d) Arrange the following in the decreasing order of their acidic strength and give reason for your answer. CH;COOH, CICH-COOH, FCH,COOH, C«H;COOH
e) Identify A and B of the following reactions.
OR
Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group which may respectively be called alkyl amines and aryl amines.
a) Give a test to distinguish alkyl amine and aryl amine.
b) How can you separate ethylamine and dimethylamine present in mixture byHoffmann's method? (3)
c) Arrange the following amines In terms of increasing order of basic strength. Propyl amine,ethyl methylamine, trimethylamine.
d) Convert: ethanamine to methanamine
22. a. How are cement classified on the basis of hardening and setting behavior?
b. Differentiate between
i. paper and pulp
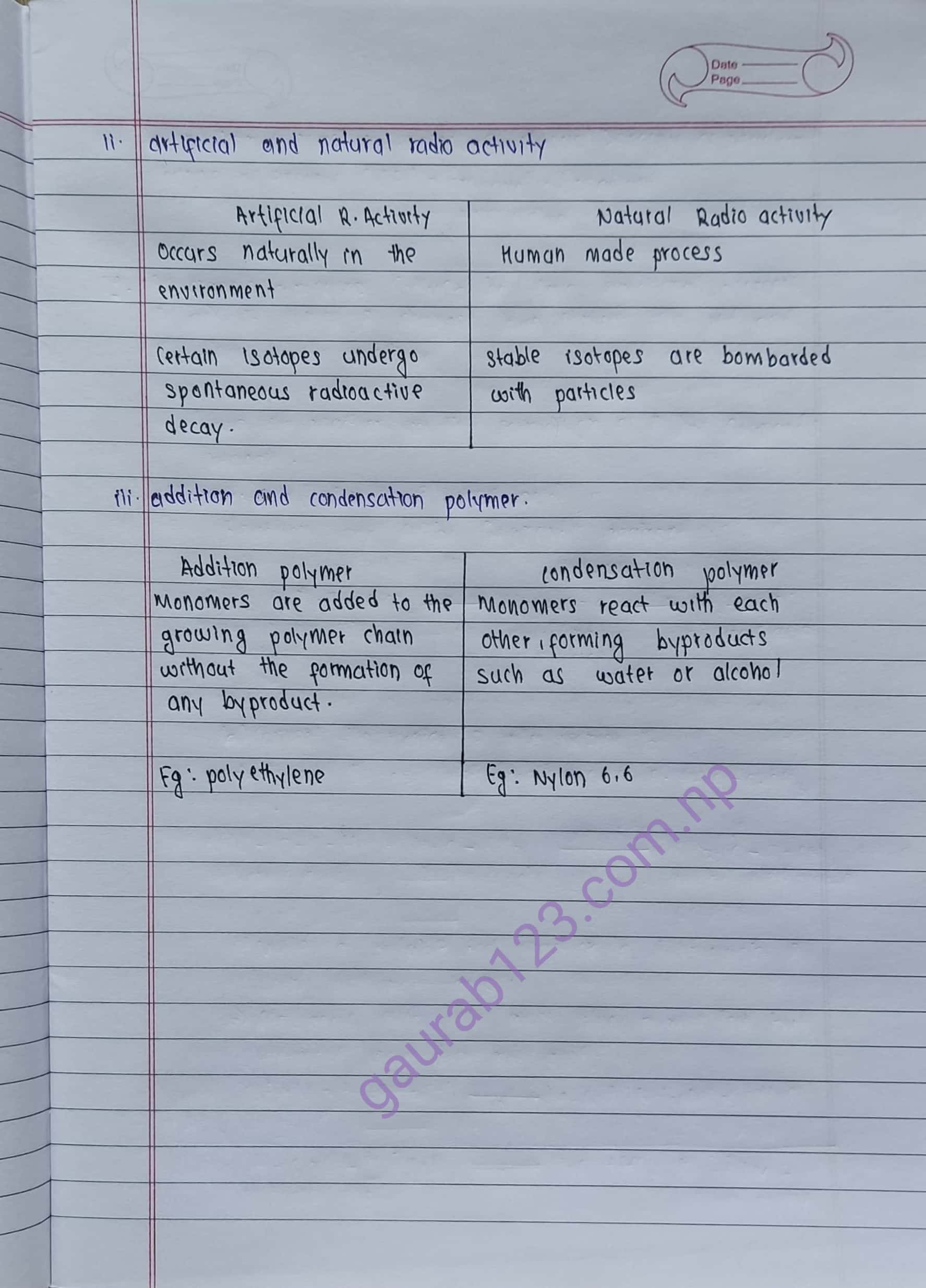
ii. artificial and natural radio activity
iii .addition and condensation polymer
SOLUTION
Rewrite the correct options of each questions in your answer sheet.
GROUP - "A "(11x1= 11)
1. Identify the equivalent weight of K2Cr2O7, in the following reaction?
(Cr =52, K=39)
K2Cr207 + 3H2SO4 + 5(COOH) => K2SO4 + Cr2(SO4)3 + 10C02 + 8H20
A) 49
B) 294
C) 98
D) 108
2.In a solution that is at equil librium. what happens to the concentration of H+ ions if the concentration of
OH- ions is increased?
A) The concentration of H+ ions increases
B) The concentration of H+ ions decreases
C) The concentration of H+ ions stay the same
D) It depends on the initial concentration of H+ ions
3. Assuming the rate of a reaction is doubled for every 10°C rise in temperature how many times increases the rate of temperature rises from 10°C to 100°C?
A) 112 times
B) 400 times
C) 512 times
D) 614 times
4. forthe given reactions
i. C + 02- CO2, H= - × KJmol
ii. 2C0 + 02- 2C02, H = - y KJmol
'The enthalpy of formation of CO becomes
A) 2y-x
B) 2x-y
C)(y-2x)/z
D) (x-2y)/z
5. What product would be obtained if red hot copper wire reacts with steam?
A)CuO
B) Cu2O
C) Cu202.
D) Cuo2?
6. For which manufacturing process, Bessemer converter is used?
A) Pig iron
B) Steel
C) Wrought iron
D) Cast iron
7. When Sodium phenoxide reacts with methyl bromide it gives
A) Cresol
B) Toluene
C) benzene
D) Anisole
8. Identify the X in the following reaction:
A) Na›S
B) Sn/HCI
C) LiAIH.
D) Na/Ha
9. Which of the following reagents can be used to distinguish between a phenol and a carboxylic acid?
A) КОН
B) Na
C) NaOH
D) NaHCO3
10. The colorless sweet smelling liquid compound A which exposed in air forms poisonous phosgene and also react with acetone gives sleep-inducing drug. Predict the product when the compound A reduced in a neutral medium?
A) Methylene chloride
B) Methane
C) Ethyne
D) Ethane
11. Oxygen containing organic compounds upon oxidation forms a carboxylic acid as the major organic product with its molecular mass higher by 14 units. Identify the organic compound.
A) A primary alcohol
B) An aldehyde
C) A ketone.
D) A secondary alcohol
Group B
Short answer questions
(8x5= 40)
12. The addition of solution of required concentration in a reaction mixture yields profitable products and saves reactants.
a) A solution of HCI is labelled 2M. Clarify its meaning?
b) In which aspects molarity is different frommolality?
c) List the significance of normality factor in preparation of standard solution?
d) Liquor ammonia kept at a corner of your chemistry lab is 25% (w/w) NHs and its specific gravity is 0.91. Find the molarity of liquor ammonia.
13. a) Write down the differences between rate of reaction and rate constant. (2)
b) For the reaction 2A+B-product, following data were obtained
Find,
i) order with respect to A and B. (1)
ii) the value of the rate constant of the reaction (1)
iii) the rate of reaction when the concentration of A and B is 0.5M and
0.4 M. respectively. (1)
Or
Four metals A, B, C and D react in the following way:
B displaces only A from solution. Only D and displaces hydrogen from IM HI solution. None of the metals will displace C from solution. Answer the followings:
i) Make the activity series of four metals with hydrogen
il) The standard potential for the following electrodes are:
C++ + 2e----> C, E°->0.76V
D+++ +e- ---> D, E°= +0.77V
a)Construct the galvanic cell by pointing out cathode and anode.
b) With IM solution of the ions, what will be EMF of cell?
c) Will the reaction occur: C++ + 2D++-->- C + D+++ Occur? Give reasons.
14. An ammonia solution is added to the sulphate of coinage metal A,the blue Precipitates (B) which appears dissolves in excess of reagent to form deep blue Solution(C). Answer the followings:
a) Identify A, B and C with sequence of chemical reaction.
b) Predict the electronic configuration of this metal A.
c) Select the suitable methods for the purification of metal A?
15. What is meant by d-d electron transitions? List the characteristics of transition metals.
16. Write down the structural formula and IUPAC name of tertiary alcohol with formula CH1o0. How would you apply Victor Meyer's method for the distinction of 1- propanol and 2- propanol ? Explain.
17. An organic compound X reacts with methyl magnesium bromide followed by acidic hydrolysis yields the compound Y. The compound Y On oxidation with acidified KMnO yields Z. All three gives positive iodoform test. Answer the followings:
a) Predict the compounds X, Y and Z with sequence of reaction and give their IUPAC names. (3)
b) Make the product by reacting Z with dilute NaOH?
OR
An organic compound(X) when heated with acetone gives hypnotic and nervous sedatives drugs and form carbonyl chloride when it exposes to air
a) Predict the organic compound (X)
b) Write the reactions for the formation of (X) from ethanol. A
c) Predict compound the new compound by treating (X) withconcnitric acid?
d) Convert (X) into acetylene.
18. a) Nitro group in nitrobenzene is meta-directing group towards electrophilic substitution reaction, why? How does nitrobenzene react with?
i) Zn/ NHACI ii) LiAI4,
b) What are the isomers of formula C2H7N?
19. Give an example of the following reactions
(a) RiemerTiemann's reaction (b) Perkin's condensation reaction
(c) Williamson's ether synthesis. (d) Cannizzaro's reaction
(e) Sandmever's reaction
Group C
Long answer questions
(3 x 8 =24)
20. The expressions of Ostwald's dilution law is, alpha= √k/c
a) Derive it.
b) What information can you obtain from this expression?
c) Will strong electrolytes obey this expression, why?
d) 0.IM ethanoic acid is 1.34% ionized. Find its dissociation constant.
Or
a) Hess' law is applied to calculate different types of enthalpy of reaction.
i) Illustrate the Hass law of constant heat summation.
il) Standard enthalpy of combustion of c (g); He (g) and CH, (g) are -394 KJ mol', -286 KJ mol' and -1300 KJ mol' respectively. Calculate enthalpy of formation of acetylene.
b) Draw the energy profile diagram of exothermic and endothermic reaction.
21. An organic compound C2H4O2 has two functional isomers A and B. Isomer A changes blue litmus into red and B has fruity smell.
a)Give the reaction for the formation of A by using Grignard's reagent.
b) Convert the isomer A into isomerB?
c) Write a suitable test reaction to distinguish A frommethanoic acid.
d) Arrange the following in the decreasing order of their acidic strength and give reason for your answer. CH;COOH, CICH-COOH, FCH,COOH, C«H;COOH
e) Identify A and B of the following reactions.
OR
Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group which may respectively be called alkyl amines and aryl amines.
a) Give a test to distinguish alkyl amine and aryl amine.
b) How can you separate ethylamine and dimethylamine present in mixture byHoffmann's method? (3)
c) Arrange the following amines In terms of increasing order of basic strength. Propyl amine,ethyl methylamine, trimethylamine.
d) Convert: ethanamine to methanamine
22. a. How are cement classified on the basis of hardening and setting behavior?
b. Differentiate between
i. paper and pulp
ii. artificial and natural radio activity
iii .addition and condensation polymer
Class 12 Chemistry Model Question 2023 Solution || Chemistry Model Question Solution class 12 || Model question 2023 class 12 chemistry solution || Chemistry 2023 model question solution || class 12 chemistry new model question 2023 || class 12 chemistry new model question solution || class 12 chemistry new model question solution 2023 || 2079 new model question of chemistry solution || Model question class 12 solution || Class 12 chemistry || Class 12 chemistry model question solution 2079 || Class 12 Chemistry Model Question 2023 || Class 12 Chemistry Model Question 2079 || Board Exam chemistry class 12 2080 || class 12 model questions || class 12 new model question chemistry || Model question solution.































Organic Chemistry ko answer haru dherai wrong xa tei vayera ramro sanga hera garam hai sathi haru
ReplyDeletekun question..mention please
DeleteQuestion no 17 and 21b
ReplyDeleteIf you have any doubts, Please let me know